Cyclotron and Radiopharmaceuticals Department (C&RD) is a state-of-the art facility for two primary activities: Radiopharmaceuticals manufacturing, and Radioisotope research. Radiopharmaceuticals manufacturing program is not only quite unique even for a major medical center, but also an essential component of KFSH&RC in providing the specialty services and quality patient care for the population of the Kingdom. On-demand and reliable availability of radiopharmaceuticals are essential components of viable nuclear medicine practice. C&RD manufactures a wide range of radiopharmaceuticals used in diagnostic medical imaging, as well as for radiotherapy. Products are manufactured according to the international and Saudi Food and Drug Authority (SFDA) standards of Good Manufacturing Practices (GMP), as well as in adherence to the ISO 9001 Quality Management System (QMS). The high-quality products are supplied to some 50 nuclear medicine centers throughout the Kingdom.
Radiopharmaceuticals manufactured at the C&RD facility are characterized by the relatively short to ultra-short half-life of the radioisotope contained within. Therefore, the optimum utilization of these radiopharmaceuticals necessitates on-site availability of a particle accelerator (cyclotron) which can transform non-radioactive atoms into radioactive atoms. At the C&RD facility, three cyclotrons are currently dedicated to producing several essential radioisotopes, which are transformed into radiopharmaceuticals, and subsequently qualified as suitable for human use through rigorous testing and quality assurance.
Research activities of the C&RD are focused on developing new radioisotopes and radioactive molecular probes with potential usefulness in molecular imaging and therapy. The facility comprises of appropriate equipment, including a micro-PET/CT for in vivo studies of the new radiotracers for achieving translational research from bench to the bed-side.C&RD's multi-disciplinary staff is a blend of engineers, chemists, radiochemists, Radiopharmaceutical, and quality control chemists all working toward KFSH&RC's goal of providing the specialty services in patient care. The Department is committed to the policy of Quality in Performance and Pride in Achievement.
Focus Areas
Radiopharmaceutical’s manufacturing
Radioisotope research
Introduction to Particle Accelerators A radioactive substance can be formed by bombarding a stable substance with charged particles in a linear or circular type accelerator. The tools most frequently used are circular accelerators, also called cyclotrons. But whatever the technology used, the same type of radionuclide is formed by the bombardment of an identical target with the same type of charged particles. The cyclotron is one of the earliest types of particle accelerators, and is still used as the first stage of some large multi-stage particle accelerators. Lawrence and M.S. Livingston invented the cyclotron in 1930.

Main Parts of a Cyclotron
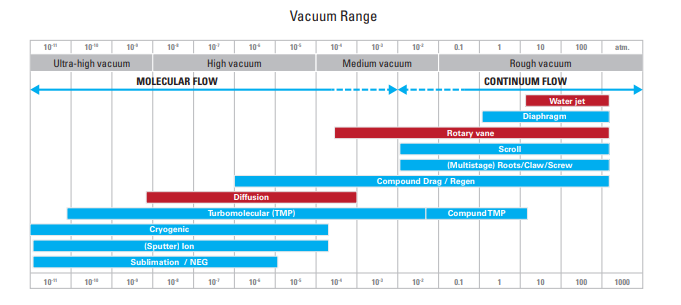
Vacuum
Particles can only be accelerated inside a relatively high vacuum & ultra-high vacuum.
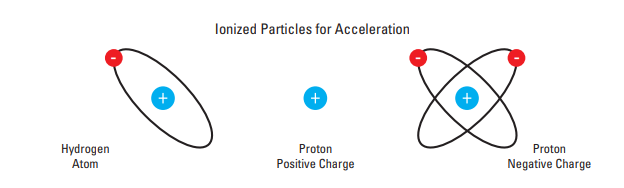
Ion Source
•Produces ionized particles (protons) for acceleration. •
•These can be of negative charge, similar to what we have in C-30 & RDS-111, or the can be of positive charge like CS-30 cyclotron.
Acceleration Force (RF)
An alternating radio frequency (RF) is used to pull/push ions to higher velocities within a magnetic field.
Main Coil
A magnetic field forces the particles to travel in a spiral path. Thus, in a cyclotron, charged particles of low mass, such as protons, are accelerated in a circular trajectory until they reach high energy. These particles are used to bombard a specific target which may be solid, liquid or gas, transforming it into a radioactive material. Unlike a reactor, a cyclotron is ideal when radioisotope formed is an element different from the cold isotope serving as the target. Thus Fluorine 18, a positron emitter, is formed from Oxygen 18, a stable isotope available in the form of a water molecule in liquid form. Iodine 123, a γ emitter, is produced from Xenon 124, a stable gaseous isotope. At the end of the process, the radioactive element can easily be separated from the cold element that served as a target. In a cyclotron, the particles are accelerated in a circular electromagnetic field under a high vacuum. When these particles reach a suitable speed, the beam is directed outside the field onto the target to be irradiated. The operation hardly lasts a few hours. The irradiated target is extracted from the cyclotron in order to be treated by radiochemists to separate the radioactive elements from the residual cold matter. Products obtained via a cyclotron have an extremely high specific activity. In fact, the products are virtually pure, but the quantity of material actually available can be counted in millionths of a milligram.


Safety Systems of the Cyclotrons Facility
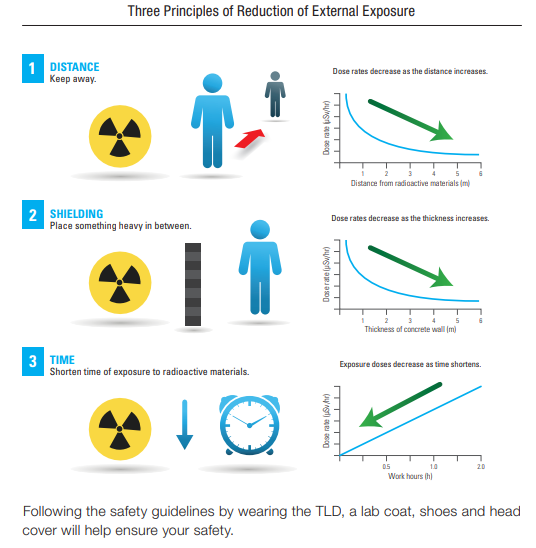
In addition to the self-safety system, which operates within the PLC-technology included in each cyclotron, the facility is equipped with a radiation monitoring system, which can detect the radiation levels inside the rooms or corridors. Safety First ALARA (As Low As Reasonably Achievable) is a safety principle designed to minimize radiation doses and releases of radioactive materials. More than merely best practice, ALARA is predicated on legal dose limits for regulatory compliance
Dose Reduction
RADIOCHEMISTRY SECTION
Summary of Hot Cells Laboratory
Hot Cell
•Hot cell components
•Hot cell unit
•Manipulators
•Liquids hot waste drain
•Air filtration & circulation
•Lead glass window
•Conveyor belt
•Dose calibrator and balance
•Target sending & receiving unit
•Glassware transfer unit
•Hot cell control panel
Hot waste disposal
•Liquid hot waste
•Solid hot waste
•Used copper targets
•Fume hood cabinet
•Chemicals storage
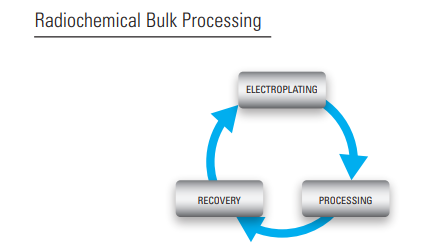
Recovery & Electroplating Laboratory Recovery:
Recovery: Collecting the enriched target material from several used targets to be reused in next electroplating. Electroplating process: Depositing the enriched material on copper plate using electrolytic cell.
• Recovery process.
• Electroplating solution preparation.
• Electroplating unit.
•Iodine-123 Concentration Unit

Good Manufacturing Practices (GMP): Standards and Requirements
Definition
The GMP regulations establish minimum standards for the manufacturing of medicinal products to assist in preventing adulteration. Patients expect that each batch of medicines they take will meet quality standards so that they will be safe and effective.
GMPs provide for systems that assure proper design, monitoring, and control of manufacturing processes and facilities. Adherence to the GMP regulations assures the identity, activity, quality, and purity of radiopharmaceutical products.
Importance
• Government requirement
• Ensures product Quality as a result of comprehensive “Quality by Design” concept
• Reduces rejects and recalls
• Maintain manufacturing consistency
• Satisfied customers
• Entity image and reputation
It is important that radiopharmaceuticals are manufactured under conditions and practices required by the GMP regulations to assure that quality is built into the design and manufacturing process at every step.
Ten Principles of GMP Lifestyle
•There are ten good manufacturing principles that we believe will help in achieving a “GMP lifestyle” in our Cyclotron department.
•Writing step-by-step procedures and work instructions.
•Carefully following written procedures and instructions to prevent contamination, mix-up, and errors.
•Accurately document work using Document Management System.
•Validating our work by following a Master Validation Plan.
•Integrating productivity, product quality, and employee safety into the design and construction of our facility and equipment.
•Properly maintaining our facilities and equipment.
•Clearly defining and demonstrating job confidence.
•Protecting our products against contamination, by practicing cleanliness and hygiene guidelines
•Building quality into our products by systematically controlling our components and product-related processes, such as manufacturing, packaging, labeling, testing, distribution, and marketing.
•Conducting planned and periodic audits for compliance and performance using the Audit Management System
Aseptic Technique, Including Gowning
Aseptic technique means using practices and procedures to prevent contamination from pathogens. Sterile compounding involves the dilution, mixing, and dispensing of various products using aseptic technique. Failure to follow the protocol of aseptic technique may lead to microbial contamination to the radiopharmaceutical product.
Aseptic Garbing, Hand Washing, and Gloving
•Washing forearms and hands using and appropriate antimicrobial agent
•Personnel may cleanse their hands or gloves with sterile 70% isopropyl Alcohol (IPA).
•A sterile gown and a pair of sterile gloves.
•They should wear closed-toe shoes because of the potential of injury by needles or broken glass. Also, they need to place disposable shoe covers over close-toed shoes
•Head covers and face maskTechnicians must assess their physical appearance for any violations. These violations are including and not limited to:
•Wearing cosmetics, hair spray, perfumes, artificial nails or nail polishing
•Wearing jewelry including body piercing not covered with a gown and mask
•Dirty and long fingernails
•Presence of weeping sores, rashes, sunburns or respiratory infection Personnel protective equipment (PPE) are used to minimize the risk of contamination of a sterile compounding area and the •Compounded Sterile Preparation (CSP)s. Cleaning the hood bench, preparation of dispensing machine and vials labeling should follow the precautions of aseptic technique
Cleaning and Disinfection of Work Areas
Bacterial and fungal spores are one of the most pervasive and resilient microorganisms on earth. By definition, a sterile environment is 100% free of all microorganisms, including spores. Disinfection methods may slow, disrupt or hinder the proliferation of microorganisms; however disinfection is not considered sporicidal. Sterilization describes a process that destroys or eliminates all forms of microbial life and is carried out in health-care facilities by physical or chemical methods. Steam under pressure, dry heat, EtO gas, hydrogen peroxide gas plasma, and liquid chemicals are the principal sterilizing agents used in health-care facilities. When chemicals are used to destroy all forms of microbiologic life, they can be called chemical sterilant. These same germicides used for shorter exposure periods also can be part of the disinfection process (i.e., high-level disinfection). Disinfection describes a process that eliminates many or all pathogenic microorganisms, except bacterial spores, on inanimate objects.
Depyrogenation and Steam Sterilization
Steam Sterilization Autoclaving with steam is the method of choice for most applications. Presence of moisture with heat denatures proteinaceous materials. Saturated steam enters the chamber, condenses on product, imparts latent heat energy to product. As temperature/pressure is increased, time required to kill is significantly reduced.
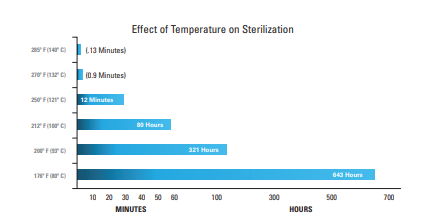
Dehydrogenation
Heat is produced by electrical heating coils. Introduced into the chamber at atmospheric pressure. Fans circulate the heated air. The process consists of heat up, exposure and cooldown. Temperature in the chamber is raised to approximately 220°C (482°F). Dry heat is not as efficient as steam. Mode of kill is an oxidation process rather than coagulation. It is the preferred method for dehydrogenation of glassware in the pharmaceutical industry
Assay Measurement Using Dose Calibrators
An ionization chamber is an instrument constructed to measure the number of ions within a medium. Ionization chambers are used in nuclear medicine to determine the exact activity of radioactive dose to be administered to the patients. Such devices are called “radioisotope dose calibrators”.
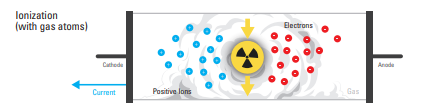
• Detectors are filled with gases such as inert gases or air.
• When radiation passes through gas, molecules are ionized, creating positive ions and electrons.
• Positive ions and electrons are drawn toward the electrodes and are converted into electric signals for measurement. Examples: GM counter survey meters, ionization chambers, etc.
• When radiation passes through a scintillator, molecules are excited, but they return to their original state (ground state).
• Light emitted in the process is amplified and converted into a current for measurement
. Examples: Nal(TI) scintillation survey meter, etc
Measurements are carried out utilizing the interaction between radiation and substances. Geiger Muller (GM) counter survey meters and ionization chambers utilize the ionization between radiation and gas atoms. NaI (Tl) scintillation survey meters utilize excitation with substances. It is essential to perform the following test procedures correctly since patient safety is highly dependent upon the reliability of this instrument.

Packaging of Radiopharmaceutical Products
Primary Packaging Materials Borosilicate Glass: Glass has good radiation resistant properties, although often radiation will cause discoloration. Forms of glass, such as lead glass, are used heavily in the radioactive products manufacturing industry (the yellow colored glass windows on hot cells). Glass can usually tolerate several kGy (kilogray) of absorbed dose. Halo butyl Rubber: Commonly, rubber stoppers on sterile products are made from halo (chloro or bromo) butylrubber. Published data show that 25 kGy is acceptable, but at 50 kGy the material shows signs of a breakdown in its physical properties. Secondary Packaging Materials The secondary packaging of radiopharmaceuticals and radioactive medical devices are always the shielding. They must always comply with the International Atomic Energy Agency (IAEA) standards. Read about Type A package requirements for more information. All radioactive products are considered class VII dangerous goods
Raw Materials Preparation and Management
The pharmaceutical industry needs utmost care and precision in each and every aspect of the field ranging from collecting the raw materials to getting the final product ready for supply in the market.
The pharmaceutical raw materials used for excipients include solvents and other carriers which are capable of carrying the actual drug. This excipient should not affect the chemical features of the radioisotope. Raw materials used for pharmaceutical packaging include plastic & polymers, glass, paper, aluminum foil, paper boards, etc

What is Inventory Management?
Inventory management is the process of keeping track of all the goods in stock.
PET SECTION
A Positron Emission Tomography (PET) Radiopharmaceutical (also known as PET Radiotracer) is a positron-emitting radiopharmaceutical used in positron emission tomography. Each radiotracer consists of positron emitting isotope (radioactive tag) bound to an organic ligand (targeting agent). The ligand component of each radio tracer interacts with a target, resulting a characteristic distribution of the tracer throughout the tissues. Positron emitting isotopes are short-lived and there are four positron emitting radioisotopes that are considered the biologic tracers: Carbon-11, Nitrogen-13, Oxygen-15, and Fluorine-18. 11C (t1/2 20.4 min), 15O (t1/2 2.1 min), and 13N (t1/2 10 min) are referred to as the essentials of life. Fluorine-18 (110 minutes half-life) is the most widely used. Other positron emitters include Gallium-68, Iodine-124 and Copper-64.
Introduction to PET
PET is a noninvasive diagnostic technique that provides images of the distribution of radiopharmaceuticals labeled with positron-emitting radionuclides inside the body, thereby making it possible to visualize different physiological or physiopathological processes in vivo. Positron emitters decay by emitting a positively charged electron (the positron β+) which travels a short distance before encountering an electron and the two particles annihilate to produce two 511-keV gamma rays which are emitted at 180° to each other and these are detectable using a PET imaging camera. Modern PET cameras are now coupled with CT to give accurate anatomical details, as well as enabling identification of tumors even smaller than 1cm.
Production of PET Radioisotopes
PET radioisotopes are produced in cyclotrons. A cyclotron is a particle accelerator. It is an electrically powered machine which produces charged particles in an ion chamber in the center of the machine. These particles are focused onto a ‘target’ or starting material and the bombardment causes the production of the desired radioisotope. Below are the principal nuclear reactions employed to produce various PET isotopes
Common PET Radioisotopes
The most suitable positron-emitting radionuclides for use in PET studies are 11C, 13N, 15O and 18F. The first three have a short radioactive half-life, which limits their possibility for use in centers located far from the isotope production site. In contrast, 18F is more suitable for distribution since it is more stable as a radioisotope. This feature has caused labeling with 18F to be the most widely-used option in the manufacture of radiopharmaceuticals labeled with positron-emitting radionuclides for PET.
Fluorine (18F)
The best radionuclide for clinical application in routine diagnostic PET procedures is 18F. The 18F positron-emitting energy is only 0.64 MeV—the lowest of all the positron emitters used in PET. This implies less patient radiation exposure and improved diagnostic image resolution. Furthermore, 18F decay does not involve the emission of gamma radiation that can interfere with photon detection or of particles (β- or α) that may constitute an increase in the dose of radiation received by the patient. The radioactive half-life of 18F (110 minutes) permits the transport and distribution of 18F-labeled radiopharmaceuticals to satellite centers and hospitals distant from the production site. This half-life makes it possible to complete complex syntheses and PET protocols with a duration of several hours, thereby allowing pharmacokinetic studies and analyses of metabolites.
Manufacturing of PET Radiopharmaceuticals
PET Radiopharmaceuticals are manufactured according to guidelines on ‘’Current Good Manufacturing Practices of Radiopharmaceuticals’’. Sterile disposable kits and pharmaceutical grade materials are used in PET radiopharmaceutical production. PET radiopharmaceuticals are manufactured in cleanrooms (Grade B and C) and dispensed aseptically in (Grade A) cells or hoods. Manufacture of the PET radiopharmaceuticals is generally automated because of the high activities needed. Automated synthesizers (Tracer lab Mx and Neptis, etc.) are used for manufacturing of various PET radiopharmaceuticals (FDG, FCH and FDOPA etc.). Automated synthesizers provide highly reproducible synthesis operation and compliance to cGMP.
Packaging and Transportation of PET Radiopharmaceuticals
PET Radiopharmaceuticals (multi dose vials) are labeled and packaged in compliance with regulations. As part of packaging regulations the inner container and outer shielding are labeled with radiation symbol, Radiopharmaceutical name, dosage units and volumes at calibration time. All radioactive packages include Radioactive l, ll or lll label with Transport Index (T.I.) Number. Radiation-safe packaging is an obvious requirement during the transport of radioactive pharmaceuticals. Type A packaging is an international regulation issued by the International Atomic Energy Agency (IAEA). Transportation of radiopharmaceuticals or radioactive materials must strictly comply with IAEA standards.
QUALITY CONTROL SECTION
Quality Control (QC) is a procedure or set of procedures intended to ensure that a manufactured product or performed service adheres to a defined set of quality criteria or meets the requirements of the client or customer. Radionuclide Identification Shelf-life The shelf-life (expiry period) of a radiopharmaceutical preparation depends principally on the physical half-life of the radioisotope, the radiochemical stability and the content of longer-lived radionuclidic impurities in the preparation under consideration. Many radiopharmaceuticals preparations contain radioisotopes with very short half-lives and such preparations therefore have very short shelf-lives. Such preparation requires an expiry date and time to be indicated. For example, technetium based preparations and PET preparations are normally intended to be used within less than 12 hours (some within minutes) of preparation. • At the end of the expiry period, the radioactivity will have decreased to the extent where insufficient radioactivity remains to serve the intended purpose or where the dose of active ingredient must be increased so much that undesirable physiological responses occur. Furthermore, chemical or radiation decomposition may have reduced the radiochemical purity to an unacceptable extent. Moreover, the radionuclidic impurity content may be such that an unacceptable radiation dose would be delivered to the patient. • The shelf-life of a multi-dose radiopharmaceutical preparation, after aseptic withdrawal of the first dose, will also depend on microbiological considerations. For radiopharmaceutical preparations containing radio-isotopes with long half-lives, microbiological considerations may take precedence over those based on the physical half-life of the radioisotope. For example, once the first dose has been aseptically withdrawn from a multi-dose container of an iodine-containing injection, the container should be stored at a temperature between 2–8 °C and the contents used within 7 days.
Radionuclidic Purity
Radionuclidic purity is the ratio, expressed as a percentage of the radioactivity of the desired radionuclide of the total activity of the source. For Fluorine-18, the radionuclidic purity is expressed in terms of gamma emissions 511 and 1022 KeV as a percentage of total radioactivity
pH Test
The pH test is one of the requirements for releasing radiopharmaceuticals as a final product. It might be measured either with pH meter or by pH paper. pH specification for PET products is between 4.5–7; for other radiopharmaceuticals as well, the pH range should be within the physiological human body pH. A pH meter may also be required for other purposes for testing reagents and other raw materials


HPLC Technique
HPLC (High Performance Liquid Chromatography) can be used both to measure radiochemical purity in quality control testing as well as for the assay of active component. HPLC is also useful for validation studies and for development of new products
GC Technique
GC (Gas Chromatography) is utilized for quantitation and identification of the residual solvents (ethanol, acetonitrile and other components) in various PET products. The test is performed with a gas chromatograph equipped with flame ionization detector (FID) and appropriate column (capillary or packed column). A computer with software to identify and quantitate the residual solvents is a useful feature and is generally supplied with the equipment
Osmolality
Osmolality is the measure of solute concentration per unit mass of solvent. Ideally, the isotonic range is 250–350 mOsm/kg. The equipment should be calibrated with known standard prior to use

Final Package Inspection
Ensure that the packaging conforms to that prescribed for each product. Reserve samples are maintained
Quality Assurance (QA) is an all-encompassing, independent unit, which assumes authority and responsibility for day-to-day implementation and maintenance of the Quality Management System of the C&R Department. QA is responsible for ensuring that all radiopharmaceutical products manufactured are of the desired quality and specifications, following the guidelines of good manufacturing practices as laid out by the local regulatory authority (SFDA). This is done by ensuring that all production operations are carried out by following processes which have been validated, approved and documented. Additionally, QA ensures that all materials used in the production of any batch have been qualified. Similarly, QA ensures that all equipment used in production and testing are qualified and calibrated. Also, all analytical methods should have been validated. Many critical functions and operations generally fall under the umbrella of QA, which include: Preparation of Documents for Routine Production QA is responsible for issuing batch records, QC records and labels for routine radiopharmaceutical production. QA ensures correctness of lot numbers, manufacturing, calibration and expiration dates, as well as correct information on product labels. Additionally, QA ensures that the current versions of all forms and records are used in batch manufacturing. The detailed instructions are provided in SOP # 11-02-002 and 11-02-003. Final Approval and Release of Radiopharmaceutical Products All records are brought to QA at the end of production and testing for review. QA reviews all records for correctness and accuracy, assures that all processes have been carried out as per approved procedures, and approves the product for release for patient use. The steps involved in the release of products is outlined in SOP # 07-02-001. Raw Materials Control QA ensures that all materials used in radiopharmaceutical production are of the required specifications and quality. All QC testing records (and preparation records, if any) are brought to QA, who reviews all records for correctness and accuracy, assures that all preparation and testing has been carried out as per approved procedures, and releases the material for patient use. QA also issues the requisite labels. The steps involved in the release of products are outlined in SOP # 05-02-002. QA is also responsible to ensure that all materials are acquired from approved vendors which have been qualified, and that these materials have been validated and found to be fit for purpose, as per SOP # 05-03-001
Control of Documents
QA is responsible for the management of all official documents within the C&R Department, as per SOPs # 01-02-001 to 01-02-006. Control of Documentation includes: • Review and approval of new SOPs, batch production records and testing records; • Revision and approval of existing SOPs, batch production records and testing records; • Ensuring that copies of only the current SOPs are available at the pointsof-use; • Maintaining document indices, numbering system and revision numbers, as well as historical documents; • Managing electronic storage, scanning, archiving and disposal of documents (SOPs # 11-04-001 and 11-04-002).
RESEARCH AND DEVELOPMENT SECTION
Development of Radiotracers in Research A radioactive tracer is a chemical compound in which one or more atoms have been replaced by a radioisotope. Monitoring its radioactive decay, a radiotracer can be used to explore the mechanism of chemical reactions. They are also used for flow visualization through different technologies, such as Single Photon Emission Computed Tomography (SPECT) and Positon Emission Tomography (PET). There is an increasing role for PET and SPECT in oncology, particularly as a component of early phase clinical trials. As a non-invasive functional imaging modality, PET and SPECT can be used to assess both pharmacokinetics and pharmacodynamics of novel therapeutics by utilizing radiolabelled compounds. Any biological target that is present at increased or decreased levels in cancer cells can be visualized by PET and SPECT. Ideally the target should be as specific as possible for the disease process, and consideration should also be given to the clinical information that might be gleaned from imaging the target or pathway. The development of a targeted radiotracer involves the synthesis of an extensive library of potential compounds for a particular target, with the expectation that only a few imaging agents will successfully progress to clinical PET and SPECT studies. This library can contain several analogues of the parent compound that have a known affinity for a target and often may be based on previously known compounds. The researcher groups interesting to the potential tracers must have subnanomolar or nanomolar affinity for the physiological target so as to not interfere with normal biological function. Other desirable characteristics of radiotracers for somatic tumours include:
• high specificity and/or selectivity.
• high plasma clearance and low plasma protein binding.
• neutral and hydrophilic to enhance elimination and reduce effective (radiation) dose.
• limited or measurable metabolism and amenable to kinetic modelling.
• low toxicity.
• good in vivo stability because of low metabolic clearance.
Our areas of expertise in radiotracer research include:
Development of new radiotracers, including the production such as (18FLT, 18F-choline, 18F-DOPA, 68Ga-DOTA-PSMA and 177Lu-DOTA-PSMA). • Radiolabelling by way of radiolabelled synthons. • Metal ligand conjugation, conjugate evaluation and radiometal labelling development. • Modifying pharmaceutical structures and synthesising small compound libraries for enhancing and optimising radiotracer properties including absorption, distribution, metabolism, clearance and toxicity. • Development of automated procedures for the production
List of Services Provided:
Radiopharmaceuticals manufacturing
Training and education
Research and development
Career Opportunities
• Chairman, Cyclotron And Radiopharmaceuticals
• Senior Scientist
• Cyclotron Engineer II
• Cyclotron Engineer III
• Cyclotron Engineer Technician
• Cyclotron Machinist I
• Cyclotron Machinist Technician
• Quality Control Chemist I
• Quality Control Chemist II
• Quality Control Chemist III
• Radiochemist I
• Radiochemist II
• Radiochemist II
• Radiochemist III
• Radiopharmacist II
Radiopharmacist III
• Senior Technical Specialist
• Technical Specialist
• Technical Assistant, Research Center
• Research Assistant
• Research Technician
• Hospital Assistant I








